Understanding the impact of substitution and deletions on aliphatic amidase using different large language models#
This tutorial demonstrates how to use PoET to score substitution and deletion variants of a sequence using aliphatic amidase (AMIE_PSEAE) as an example.
We’ll also compare substitution variant scores from PoET, ESM1b, and a site-independent model (PSSM) with activities assayed in a deep mutational scanning study by Wrenbeck, Azouz, and Whitehead.
[1]:
%matplotlib inline
[2]:
import numpy as np
import pandas as pd
import matplotlib
import matplotlib.pyplot as plt
import scipy
from scipy.stats import pearsonr,spearmanr,kendalltau
import scipy.special
import json
import time
from mpl_toolkits.axes_grid1 import make_axes_locatable
import seaborn as sns
sns.set()
[3]:
import openprotein
import openprotein.fasta as fasta
[4]:
name = 'AMIE_PSEAE'
wt = b'MRHGDISSSNDTVGVAVVNYKMPRLHTAAEVLDNARKIAEMIVGMKQGLPGMDLVVFPEYSLQGIMYDPAEMMETAVAIPGEETEIFSRACRKANVWGVFSLTGERHEEHPRKAPYNTLVLIDNNGEIVQKYRKIIPWCPIEGWYPGGQTYVSEGPKGMKISLIICDDGNYPEIWRDCAMKGAELIVRCQGYMYPAKDQQVMMAKAMAWANNCYVAVANAAGFDGVYSYFGHSAIIGFDGRTLGECGEEEMGIQYAQLSLSQIRDARANDQSQNHLFKILHRGYSGLQASGDGDRGLAECPFEFYRTWVTDAEKARENVERLTRSTTGVAQCPVGRLPYEGLEKEA'
print('>' + name)
for i in range(0, len(wt), 80):
print(wt[i:i+80].decode())
>AMIE_PSEAE
MRHGDISSSNDTVGVAVVNYKMPRLHTAAEVLDNARKIAEMIVGMKQGLPGMDLVVFPEYSLQGIMYDPAEMMETAVAIP
GEETEIFSRACRKANVWGVFSLTGERHEEHPRKAPYNTLVLIDNNGEIVQKYRKIIPWCPIEGWYPGGQTYVSEGPKGMK
ISLIICDDGNYPEIWRDCAMKGAELIVRCQGYMYPAKDQQVMMAKAMAWANNCYVAVANAAGFDGVYSYFGHSAIIGFDG
RTLGECGEEEMGIQYAQLSLSQIRDARANDQSQNHLFKILHRGYSGLQASGDGDRGLAECPFEFYRTWVTDAEKARENVE
RLTRSTTGVAQCPVGRLPYEGLEKEA
Connect to the OpenProtein.AI API#
[5]:
with open('secrets.config', 'r') as f:
config = json.load(f)
session = openprotein.connect(config['username'], config['password'])
Create the MSA and define an ensemble prompt#
At the core of PoET is the prompt, a set of sequences that encode information about the fitness landscape of our protein of interest. A typical way to create a prompt is by constructing an MSA from homologues of the protein of interest. We support this natively via our API (see below).
OpenProtein uses an asynchronous API, where potentially long running functions return a job ID that can be used to query for completed results. The wait_until_done function can be used to poll for completion.
[6]:
# search for homologs to automatically create an MSA for the seed sequence
msa = session.align.create_msa(wt)
# msa = session.jobs.get("340d7d97-fabb-4576-b73a-7d559ef028c2")
print(msa)
msa.wait_until_done(verbose=True)
# create the prompt, set the seed for reproducibility
prompt = msa.sample_prompt(num_ensemble_prompts=10, random_seed=1)
# prompt = session.jobs.get("2197c48f-142e-4d39-9ee3-8562f415d19e")
print(prompt)
job_id='77ab5349-e2c8-4c65-8d00-74cfa9e58499' job_type=<JobType.align_align: '/align/align'> status=<JobStatus.SUCCESS: 'SUCCESS'> created_date=datetime.datetime(2024, 10, 15, 16, 15, 55, 350428) start_date=None end_date=datetime.datetime(2024, 10, 15, 16, 15, 55, 351613) prerequisite_job_id=None progress_message=None progress_counter=None sequence_length=None name='77ab5349-e2c8-4c65-8d00-74cfa9e58499' description='' project_uuid=None args={'msa_id': '77ab5349-e2c8-4c65-8d00-74cfa9e58499', 'parent_id': '77ab5349-e2c8-4c65-8d00-74cfa9e58499'} msa_id='77ab5349-e2c8-4c65-8d00-74cfa9e58499'
Waiting: 100%|███████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [00:01<00:00, 91.19it/s, status=SUCCESS]
job_id='27d50005-28bf-4229-8e79-ca33e3ca4333' job_type=<JobType.align_prompt: '/align/prompt'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 16, 15, 58, 75810) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=None sequence_length=None name='27d50005-28bf-4229-8e79-ca33e3ca4333' description='' project_uuid=None args={'root_msa': '77ab5349-e2c8-4c65-8d00-74cfa9e58499', 'parent_id': '77ab5349-e2c8-4c65-8d00-74cfa9e58499'} prompt_id='27d50005-28bf-4229-8e79-ca33e3ca4333'
Above, you can see we first create an MSA from the wildtype (wt) protein. We then create 10 different prompts based on this, using the default prompt arguments. Each prompt will meet the prompt definition parameters but contain different sequences from the MSA to create a more robust fitness score.
Run all single substitution predictions for the parent sequence#
All single substitutions can be scored using the single_site function from our poet model:
[7]:
poet = session.embedding.get_model('poet')
[8]:
future_ssp = poet.single_site(prompt=prompt, sequence=wt)
# future_ssp = session.jobs.get("cdf6f26b-c9e1-4f35-ab43-33397aad95b2")
print(future_ssp.job)
future_ssp.wait_until_done(verbose=True)
num_records=69200 job_id='60dc6d3f-5ec1-4271-a26e-5ef80d3984b0' job_type=<JobType.poet_single_site: '/poet/single_site'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 16, 46, 28, 525569, tzinfo=TzInfo(UTC)) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=0 sequence_length=None
Waiting: 0%| | 0/100 [32:15<?, ?it/s, status=PENDING]
Waiting: 100%|███████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [06:46<00:00, 4.06s/it, status=SUCCESS]
[8]:
True
[9]:
# retrieve the results for the SSP job
results = future_ssp.get()
[10]:
results_dict = {result.mut_code: result.score for result in results}
baseline = results_dict["WT"]
amino_acids = 'ARNDCQEGHILKMFPSTWYV'
scores_ensemble = np.zeros((len(wt), 20, len(baseline))) + baseline
for i in range(scores_ensemble.shape[0]):
for j in range(len(amino_acids)):
pos = i + 1
fr = wt.decode()[i:i+1]
to = amino_acids[j:j+1]
if fr != to:
code = fr + str(pos) + to
val = results_dict[code]
scores_ensemble[i, j] = val
scores_ensemble.shape
[10]:
(346, 20, 10)
Plot the single site predictions#
[11]:
scores = np.stack([results_dict[result] for result in results_dict], axis=0) - baseline
scores = scores.mean(axis=-1)
plt.title("Sequence fitness vs wildtype")
_ = plt.hist(scores.ravel(), bins=30)
_ = plt.xlabel('Log likelihood ratio (p(variant)/p(wt))');
plt.axvline(0, color='blue', ls="--");
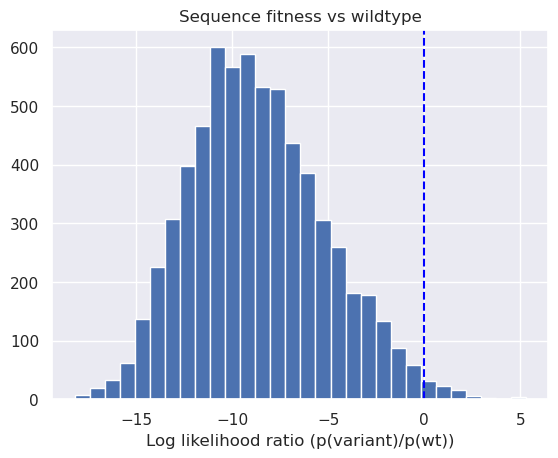
We can see above that a small subset of single site mutants have higher predicted fitness than wildtype aliphatic amidase.
[12]:
scores = np.mean(scores_ensemble - baseline, axis=-1)
fig, ax = plt.subplots(figsize=(55, 10))
plt.title("Single site mutation fitness heatmap", size=32)
# Plot the data
im = ax.imshow(scores.T, cmap='viridis')
# Set y-axis labels
y_labels = amino_acids
y_ticks = np.arange(len(y_labels))
ax.set_yticks(y_ticks)
ax.set_yticklabels(y_labels, fontsize=12)
# Label axes
plt.ylabel('Substitution', size=32)
plt.xlabel('Position', size=32)
divider = make_axes_locatable(ax)
cax = divider.append_axes("right", size="1%", pad=0.05)
plt.colorbar(im, cax=cax)
plt.colorbar(im, cax=cax)
plt.show()

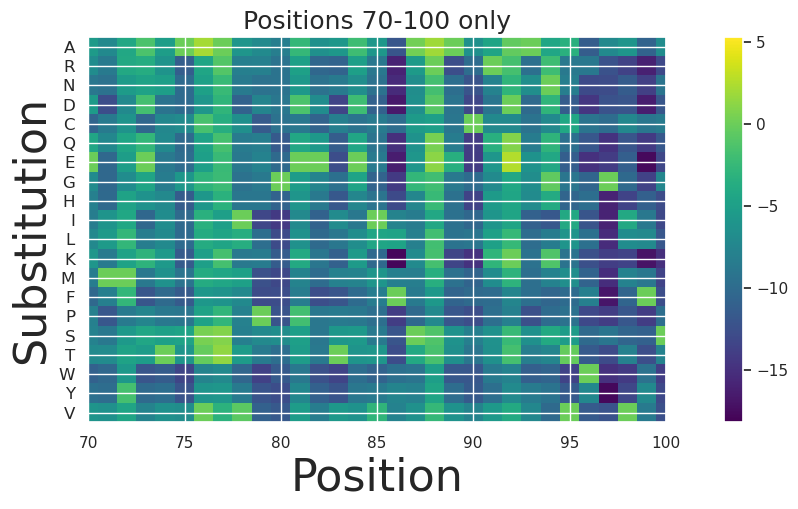
We can look at a subset of the positions more closely:
[13]:
fig, ax = plt.subplots(figsize=(15, 5))
plt.title("Positions 70-100 only", size=18)
# Plot the data
im = ax.imshow(scores.T, cmap='viridis')
# Set y-axis labels
y_labels = amino_acids
y_ticks = np.arange(len(y_labels))
ax.set_yticks(y_ticks)
ax.set_yticklabels(y_labels, fontsize=12) # Adjust fontsize as needed
# Label axes
plt.ylabel('Substitution', size=32)
plt.xlabel('Position', size=32)
plt.xlim(70,100)
plt.colorbar(im, ax=ax)
plt.show()
Plot scores from an individual prompts in the ensemble#
Because we used a prompt ensemble, we get scores for each prompt in the ensemble. They can be combined into a single score via averaging, but can also be examined individually.
[14]:
heatmap = scores_ensemble[..., 0] - baseline[..., 0]
fig, ax = plt.subplots(figsize=(55, 10))
plt.title("Single site mutation fitness heatmap (prompt 1)", size=32)
# Plot the data
im = ax.imshow(heatmap.T, cmap='viridis')
# Set y-axis labels
y_labels = amino_acids
y_ticks = np.arange(len(y_labels))
ax.set_yticks(y_ticks)
ax.set_yticklabels(y_labels, fontsize=12)
# Label axes
plt.ylabel('Substitution', size=32)
plt.xlabel('Position', size=32)
divider = make_axes_locatable(ax)
cax = divider.append_axes("right", size="1%", pad=0.05)
plt.colorbar(im, cax=cax)
plt.colorbar(im, cax=cax)
plt.show()

Rank PoET variant predictions#
[15]:
variants, scores = zip(*results_dict.items())
variants = np.array(variants)
scores = np.array(scores)
variants.shape, scores.shape
[15]:
((6575,), (6575, 10))
[16]:
# rank the variants
order = np.argsort(-scores.mean(axis=1))
for i in order[:10]:
print(variants[i], scores[i].mean(axis=-1), (scores[i]-baseline).mean(axis=-1))
A28K -156.15889599999997 5.4040289999999995
L62T -156.30312399999997 5.259801000000001
A28R -156.626914 4.936010999999998
I279L -157.308287 4.254637999999997
K93E -157.74685100000002 3.8160740000000004
Q288H -157.941995 3.620929999999997
V77A -158.30005400000002 3.2628709999999956
T327V -158.37104600000004 3.1918789999999975
R89A -158.62579300000002 2.937131999999997
L260V -158.76233899999997 2.8005860000000014
Compare the PoET substitution scores with measurements from deep mutational scanning#
Deep mutational scanning of AMIE_PSEAE has been performed by Wrenbeck, Azouz, and Whitehead. In this study, they measured activites of most single substitution variants of the wildtype protein against three substrates. Let’s load this data and see how well PoET predicted the effects of these variants compared with a few baselines.
As baselines, we’ll consider
a position-specific scoring matrix (PSSM, and additive model) fit on the MSA found above
variant effect predictions from the per-position amino acid probabilities given by ESM1b
Load the DMS data#
[17]:
path = "data/AMIE_PSEAE_Whitehead.csv"
table = pd.read_csv(path, index_col=0)
table.head()
[17]:
| mutant | acetamide_normalized_fitness | isobutyramide_normalized_fitness | propionamide_normalized_fitness | mutation_effect_prediction_vae_ensemble | mutation_effect_prediction_vae_1 | mutation_effect_prediction_vae_2 | mutation_effect_prediction_vae_3 | mutation_effect_prediction_vae_4 | mutation_effect_prediction_vae_5 | mutation_effect_prediction_pairwise | mutation_effect_prediction_independent | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | M1W | NaN | -0.5174 | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN |
| 1 | M1Y | NaN | -0.5253 | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN |
| 2 | M1P | -2.1514 | -0.5154 | -1.1457 | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN |
| 3 | M1M | 0.0000 | 0.0000 | 0.0000 | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN |
| 4 | M1I | -0.1227 | -0.3640 | -0.1212 | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN |
[18]:
cols = ['acetamide_normalized_fitness', 'isobutyramide_normalized_fitness', 'propionamide_normalized_fitness']
variants = table['mutant']
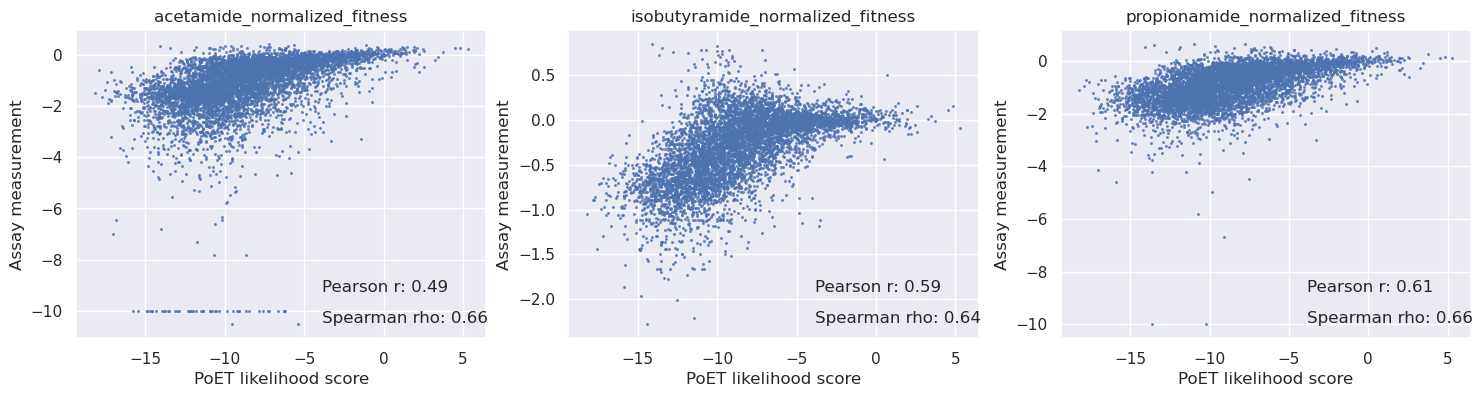
Match against the DMS variants and compare PoET log probabilities with activity measurements#
PoET log probabilities accurately predict the activity of AMIE variants for all three substrates.
[19]:
baseline = results_dict['WT']
variants = table['mutant'].values
logp = np.array([results_dict.get(code, baseline) for code in variants])
logp.shape
[19]:
(6819, 10)
[20]:
scores = np.mean(logp, axis=-1) - baseline.mean()
sigma = np.std(logp - baseline, axis=-1)
# Create subplots with labels and formatting
fig, axs = plt.subplots(1, len(cols), figsize=(6 * len(cols), 4))
for i in range(len(cols)):
c = cols[i]
y = table[c].values
axs[i].scatter(scores, y, s=1.5, alpha=0.7)
axs[i].set_title(c)
axs[i].set_xlabel('PoET likelihood score')
axs[i].set_ylabel('Assay measurement')
mask = np.isnan(y)
r = pearsonr(scores[~mask], y[~mask])[0]
rho = spearmanr(scores[~mask], y[~mask])[0]
# Add correlation values on the graph
axs[i].text(0.6, 0.15, f'Pearson r: {r:.2f}', transform=axs[i].transAxes, fontsize=12)
axs[i].text(0.6, 0.05, f'Spearman rho: {rho:.2f}', transform=axs[i].transAxes, fontsize=12)
plt.show()
Compare predictiveness of single prompts with the ensemble#
We can also check each prompts results vs the assay data to examine the variance from prompting:
[21]:
scores = np.mean(logp, axis=-1)
sigma = np.std(logp - baseline, axis=-1)
rows = []
for i in range(logp.shape[-1]):
pred = logp[..., i]
row = [f'Prompt {i+1}']
for j in range(len(cols)):
c = cols[j]
y = table[c].values
mask = np.isnan(y)
rho = spearmanr(pred[~mask], y[~mask])[0]
row.append(rho)
rows.append(row)
arr = pd.DataFrame(rows).iloc[:, 1:].values
means = arr.mean(axis=0)
rows.append(['Average'] + means.tolist())
stdevs = arr.std(axis=0)
rows.append(['stdevs'] + stdevs.tolist())
pred = scores
row = [f'Ensemble']
for j in range(len(cols)):
c = cols[j]
y = table[c].values
mask = np.isnan(y)
rho = spearmanr(pred[~mask], y[~mask])[0]
row.append(rho)
rows.append(row)
ensemble_comparison_table = pd.DataFrame(rows, columns=['Prompt'] + cols)
ensemble_comparison_table.round(2)
[21]:
| Prompt | acetamide_normalized_fitness | isobutyramide_normalized_fitness | propionamide_normalized_fitness | |
|---|---|---|---|---|
| 0 | Prompt 1 | 0.67 | 0.62 | 0.66 |
| 1 | Prompt 2 | 0.66 | 0.62 | 0.65 |
| 2 | Prompt 3 | 0.65 | 0.62 | 0.64 |
| 3 | Prompt 4 | 0.64 | 0.61 | 0.63 |
| 4 | Prompt 5 | 0.62 | 0.61 | 0.62 |
| 5 | Prompt 6 | 0.65 | 0.63 | 0.63 |
| 6 | Prompt 7 | 0.64 | 0.62 | 0.63 |
| 7 | Prompt 8 | 0.65 | 0.64 | 0.64 |
| 8 | Prompt 9 | 0.66 | 0.62 | 0.65 |
| 9 | Prompt 10 | 0.65 | 0.62 | 0.64 |
| 10 | Average | 0.65 | 0.62 | 0.64 |
| 11 | stdevs | 0.01 | 0.01 | 0.01 |
| 12 | Ensemble | 0.66 | 0.63 | 0.65 |
Having a diversity of prompts is useful in finding a more accurate mean fitness score that is less reliant on specific sequences (that might be outliers). However we can see here that the prompts built on this MSA are all reasonable similar (stdev <= 0.03).
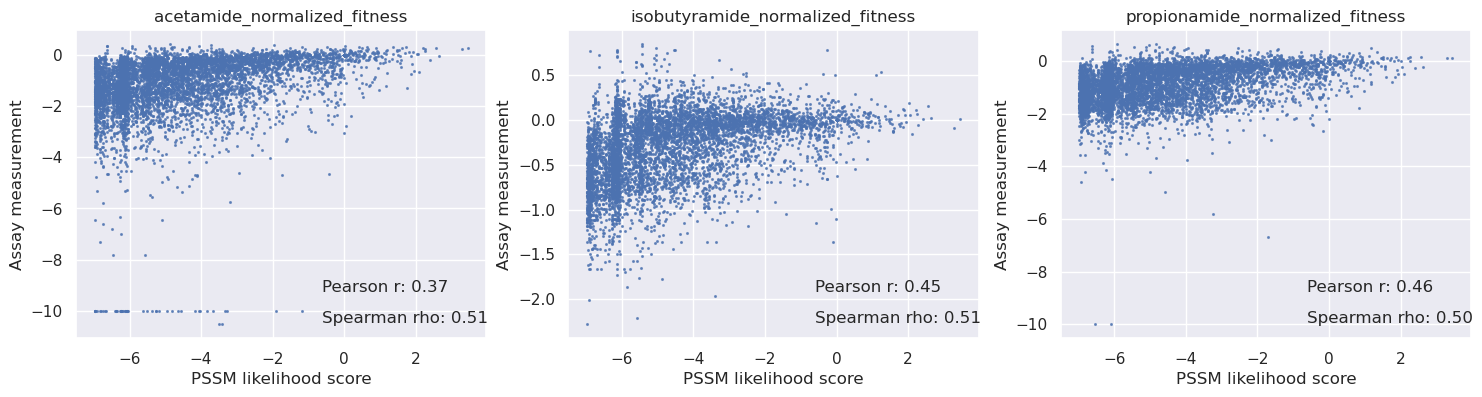
Compare the PoET scores with a PSSM and ESM1b#
We can compare OpenProtein’s proprietary model with open source models such as PSSM and ESM1b. From this comparison we find PoET is significantly more predictive, especially for acetamide and propionamide.
[22]:
# retrieve the MSA and fit the PSSM
msa_sequences = []
for name, sequence in msa.get():
msa_sequences.append(sequence)
msa_sequences = np.array([[c for c in msa_sequences[i]] for i in range(len(msa_sequences))])
msa_sequences.shape
[22]:
(1244, 346)
Calculate the PSSM scores#
[23]:
# count the amino acids at each site and calculate site frequencies with a small pseudocount
from collections import Counter
pseudocount = 1
pssm = []
for i in range(msa_sequences.shape[1]):
counts = Counter(msa_sequences[:, i])
freqs = {}
total = sum(counts.get(a, 0) + pseudocount for a in amino_acids)
for a in amino_acids:
c = counts.get(a, 0) + pseudocount
freqs[a] = np.log(c) - np.log(total)
pssm.append(freqs)
[24]:
# score the variants in the DMS dataset using the PSSM
scores_pssm = np.zeros(len(table))
# first, score the WT
logp_pssm_wt = 0
for j, aa in enumerate(wt.decode()):
logp_pssm_wt += pssm[j][aa]
for i,mut in enumerate(table['mutant'].values):
# parse the mutant sequence
wt_aa, site, var_aa = mut[:1], int(mut[1:-1])-1, mut[-1:]
assert wt_aa == wt[site:site+1].decode(), f'{wt_aa}, {wt[site]}, {site}'
s = wt[:site].decode() + var_aa + wt[site+1:].decode()
logp = 0
for j, aa in enumerate(s):
logp += pssm[j][aa]
scores_pssm[i] = logp
scores_pssm = scores_pssm - logp_pssm_wt
scores_pssm.shape
[24]:
(6819,)
[25]:
_, axs = plt.subplots(1, len(cols), figsize=(6 * len(cols), 4))
for i in range(len(cols)):
c = cols[i]
y = table[c].values
axs[i].scatter(scores_pssm, y, s=1.5, alpha=0.7)
axs[i].set_title(c)
axs[i].set_xlabel('PSSM likelihood score')
axs[i].set_ylabel('Assay measurement')
mask = np.isnan(y)
r = pearsonr(scores_pssm[~mask], y[~mask])[0]
rho = spearmanr(scores_pssm[~mask], y[~mask])[0]
# Add correlation values on the graph
axs[i].text(0.6, 0.15, f'Pearson r: {r:.2f}', transform=axs[i].transAxes, fontsize=12)
axs[i].text(0.6, 0.05, f'Spearman rho: {rho:.2f}', transform=axs[i].transAxes, fontsize=12)
plt.show()
Calculate the ESM1b scores using the API#
[26]:
esm1b = session.embedding.esm1b
[27]:
# to make the ESM1b scores, we get the probability of the amino acids at each site if the site is masked
# ESM1b will return logits flanked by start and stop token positions
queries = []
for i in range(len(wt)):
q = wt[:i] + b'X' + wt[i+1:]
queries.append(q)
future_logits_esm1b = esm1b.logits(queries)
print(future_logits_esm1b.job)
num_records=346 job_id='aff78631-5d61-4996-9891-5dc763b99e45' job_type=<JobType.embeddings_logits: '/embeddings/logits'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 17, 18, 55, 482410, tzinfo=TzInfo(UTC)) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=0 sequence_length=None
[28]:
future_logits_esm1b.wait_until_done(verbose=True)
Waiting: 100%|███████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [00:37<00:00, 2.70it/s, status=SUCCESS]
[28]:
True
[29]:
results_esm1b = future_logits_esm1b.get()
# parse the site potentials out from the results for each masked site
logits_esm1b = [None]*len(wt)
for sequence, array in results_esm1b:
# discard the leading <cls> token and the trailing <eos> token
array = array[1:-1]
# find the masked position to get the logits there
i = sequence.index(b'X')
logits_esm1b[i] = array[i]
logits_esm1b = np.stack(logits_esm1b, axis=0)
# parse the tokens
tokens = esm1b.metadata.output_tokens[:logits_esm1b.shape[1]]
esm1b_scoring_matrix = []
for i in range(len(wt)):
s = scipy.special.log_softmax(logits_esm1b[i])
site_scores = {}
for j,t in enumerate(tokens):
site_scores[t] = s[j]
esm1b_scoring_matrix.append(site_scores)
logits_esm1b.shape
[29]:
(346, 33)
[30]:
# score the variants in the DMS dataset using the PSSM
scores_esm1b = np.zeros(len(table))
# first, score the WT
logp_esm1b_wt = 0
for j, aa in enumerate(wt.decode()):
logp_esm1b_wt += esm1b_scoring_matrix[j][aa]
for i,mut in enumerate(table['mutant'].values):
# parse the mutant sequence
wt_aa, site, var_aa = mut[:1], int(mut[1:-1])-1, mut[-1:]
assert wt_aa == wt[site:site+1].decode(), f'{wt_aa}, {wt[site]}, {site}'
s = wt[:site].decode() + var_aa + wt[site+1:].decode()
logp = 0
for j, aa in enumerate(s):
logp += esm1b_scoring_matrix[j][aa]
scores_esm1b[i] = logp
scores_esm1b = scores_esm1b - logp_esm1b_wt
scores_esm1b.shape
[30]:
(6819,)
[31]:
score_map_esm1b = np.zeros((len(wt), 20))
for i in range(len(wt)):
for j in range(20):
aa = amino_acids[j:j+1]
score_map_esm1b[i, j] = esm1b_scoring_matrix[i][aa] - esm1b_scoring_matrix[i][wt[i:i+1].decode()]
[32]:
heatmap = scores_ensemble[..., 0] - baseline[..., 0]
fig, ax = plt.subplots(figsize=(55, 10))
plt.title("Single site mutation fitness heatmap (ESM1b)", size=32)
# Plot the data
im = ax.imshow(score_map_esm1b.T, cmap='viridis')
# Set y-axis labels
y_labels = amino_acids
y_ticks = np.arange(len(y_labels))
ax.set_yticks(y_ticks)
ax.set_yticklabels(y_labels, fontsize=12)
# Label axes
plt.ylabel('Substitution', size=32)
plt.xlabel('Position', size=32)
divider = make_axes_locatable(ax)
cax = divider.append_axes("right", size="1%", pad=0.05)
plt.colorbar(im, cax=cax)
plt.colorbar(im, cax=cax)
plt.show()

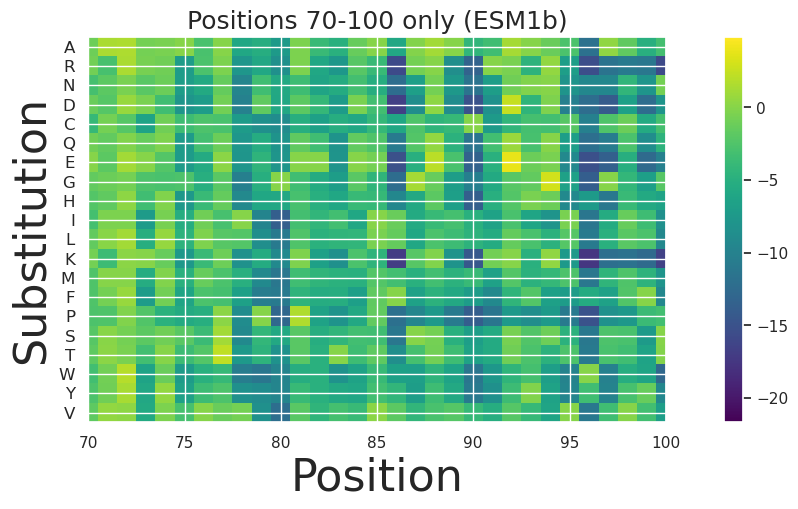
[33]:
fig, ax = plt.subplots(figsize=(15, 5))
plt.title("Positions 70-100 only (ESM1b)", size=18)
# Plot the data
im = ax.imshow(score_map_esm1b.T, cmap='viridis')
# Set y-axis labels
y_labels = amino_acids
y_ticks = np.arange(len(y_labels))
ax.set_yticks(y_ticks)
ax.set_yticklabels(y_labels, fontsize=12) # Adjust fontsize as needed
# Label axes
plt.ylabel('Substitution', size=32)
plt.xlabel('Position', size=32)
plt.xlim(70,100)
plt.colorbar(im, ax=ax)
plt.show()
[34]:
_, axs = plt.subplots(1, len(cols), figsize=(6 * len(cols), 4))
for i in range(len(cols)):
c = cols[i]
y = table[c].values
axs[i].scatter(scores_esm1b, y, s=1.5, alpha=0.7)
axs[i].set_title(c)
axs[i].set_xlabel('ESM1b likelihood score')
axs[i].set_ylabel('Assay measurement')
mask = np.isnan(y)
r = pearsonr(scores_esm1b[~mask], y[~mask])[0]
rho = spearmanr(scores_esm1b[~mask], y[~mask])[0]
# Add correlation values on the graph
axs[i].text(0.6, 0.15, f'Pearson r: {r:.2f}', transform=axs[i].transAxes, fontsize=12)
axs[i].text(0.6, 0.05, f'Spearman rho: {rho:.2f}', transform=axs[i].transAxes, fontsize=12)
plt.show()
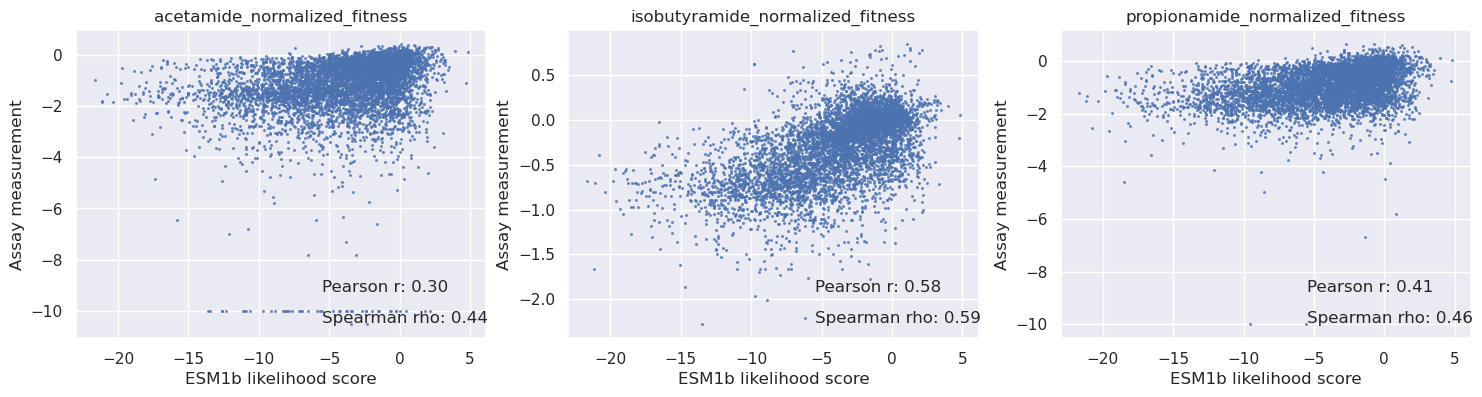
Fitness comparison summary#
We can see that the correlations between assay data and predicted fitness for acetamine, isobutyramide and propionamide with Poet are significantly better than the PSSM and ESM1b models (see the table below):
[35]:
from IPython.display import display
scores_vae = table['mutation_effect_prediction_vae_ensemble'].values
vae_mask = np.isnan(scores_vae)
score_names = ['PSSM', 'ESM1b', 'PoET']
score_arrays = [scores_pssm, scores_esm1b, scores]
rows = []
for i in range(len(cols)):
c = cols[i]
y = table[c].values
mask = np.isnan(y)
rhos = []
for j in range(len(score_names)):
rho = spearmanr(score_arrays[j][~mask], y[~mask])[0]
rhos.append(rho)
axs[i].set_title(c)
row = [c] + rhos
rows.append(row)
result_table = pd.DataFrame(rows, columns=['Property'] + score_names)
print("Correlation (assay vs model) results per property and model:")
display(result_table.round(2))
Correlation (assay vs model) results per property and model:
| Property | PSSM | ESM1b | PoET | |
|---|---|---|---|---|
| 0 | acetamide_normalized_fitness | 0.50 | 0.44 | 0.66 |
| 1 | isobutyramide_normalized_fitness | 0.51 | 0.59 | 0.63 |
| 2 | propionamide_normalized_fitness | 0.49 | 0.46 | 0.65 |
Analyze deletion variants of AMIE_PSEAE#
Single deletion screen#
Predict the deletion tolerance for each site in AMIE_PSEAE using the score function of PoET to evaluate the log-likelihoods of specified sequences.
[36]:
queries = [wt]
for i in range(len(wt)):
q = wt[:i] + wt[i+1:]
queries.append(q)
len(queries)
[36]:
347
[37]:
future_score = poet.score(prompt=prompt, sequences=queries)
print(future_score.job)
num_records=3470 job_id='80b88abb-aabf-4e39-9397-317f66854528' job_type=<JobType.poet_score: '/poet/score'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 17, 20, 48, 88544, tzinfo=TzInfo(UTC)) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=0 sequence_length=None
[38]:
future_score.wait_until_done(verbose=True)
Waiting: 100%|███████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [04:13<00:00, 2.54s/it, status=SUCCESS]
[38]:
True
[39]:
results_del_scan = future_score.get()
[40]:
scores_del_ensemble = np.stack([r[2] for r in results_del_scan], axis=0)
scores_del_ensemble.shape
[40]:
(347, 10)
[41]:
import numpy as np
import matplotlib.pyplot as plt
scores_del = np.mean(scores_del_ensemble - baseline, axis=-1)
# Create a colormap for coloring points based on height
colormap = plt.get_cmap('viridis') # You can choose a different colormap
_, ax = plt.subplots(figsize=(20, 4))
scatter = ax.scatter(np.arange(0, len(wt) + 1), scores_del, c=scores_del, cmap=colormap)
ax.set_xlim(0, len(scores_del) + 2)
ax.set_xticks(np.arange(0, len(scores_del), 50), ['WT'] + [str(i) for i in np.arange(50, len(scores_del), 50)])
plt.xlabel('Position')
plt.ylabel('PoET Likelihood Score')
plt.title('Deletion Tolerance Scan')
# Add a colorbar to indicate the values associated with the colors
#cbar = plt.colorbar(scatter, ax=ax)
#cbar.set_label('PoET Likelihood Score')
plt.show()
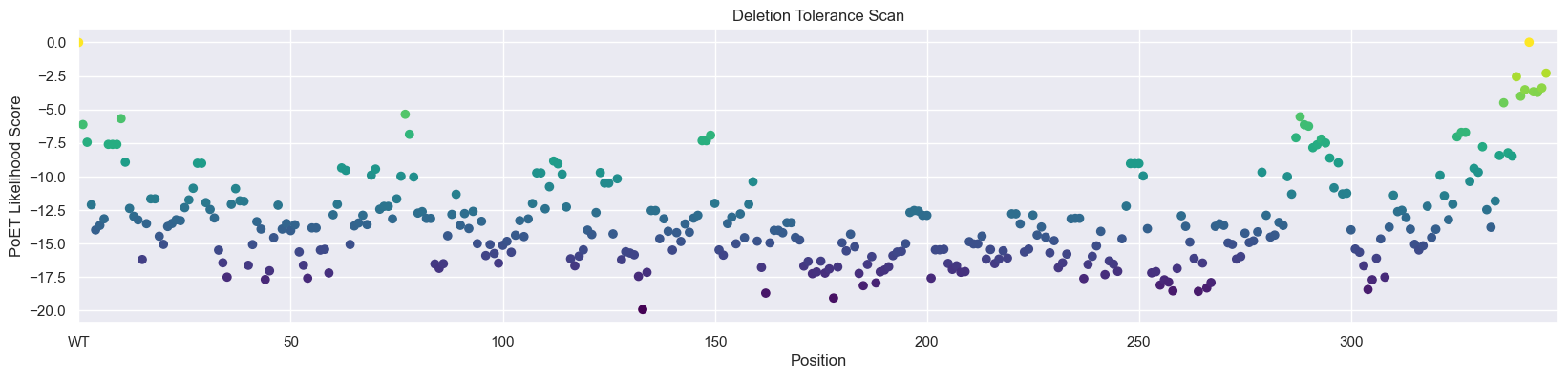
Deletion fragment screen#
Now, let’s predict the deletion tolerance for fragments (subsequence) of up to 16 amino acids by enumerating and scoring the log-likelihoods for all of them. For AMIE_PSEAE, there are about 5,400 possible such deletions.
[42]:
# enumerate WT variants with all deletions of up to size 16
# NOTE - empty string sequences are not scores by the PoET API
queries = [wt]
for i in range(len(wt)):
for j in range(i, min(i+16, len(wt))):
q = wt[:i] + wt[j+1:]
queries.append(q)
len(queries)
[42]:
5417
[43]:
# submit this in multiple chunks, because the number of query sequences is large
futures_score_del_span = []
batch_size = 2000
for i in range(0, len(queries), batch_size):
batch = queries[i:i+batch_size]
f = poet.score(prompt=prompt, sequences=batch)
print(i, 'to', min(len(queries), i+batch_size), f.job)
futures_score_del_span.append(f)
0 to 2000 num_records=20000 job_id='5f7d654f-990b-4096-8a19-21210d8ec480' job_type=<JobType.poet_score: '/poet/score'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 17, 30, 26, 859228, tzinfo=TzInfo(UTC)) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=0 sequence_length=None
2000 to 4000 num_records=20000 job_id='d46bd9a5-8fcb-4180-b573-10dd2749ecd3' job_type=<JobType.poet_score: '/poet/score'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 17, 30, 34, 522835, tzinfo=TzInfo(UTC)) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=0 sequence_length=None
4000 to 5417 num_records=14170 job_id='ac287f52-1d48-4e89-8063-6a59c4d04bc4' job_type=<JobType.poet_score: '/poet/score'> status=<JobStatus.PENDING: 'PENDING'> created_date=datetime.datetime(2024, 10, 15, 17, 30, 41, 723274, tzinfo=TzInfo(UTC)) start_date=None end_date=None prerequisite_job_id=None progress_message=None progress_counter=0 sequence_length=None
[44]:
# wait for all of the score jobs to finish
for f in futures_score_del_span:
f.wait_until_done(verbose=True)
Waiting: 100%|██████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [00:00<00:00, 390.00it/s, status=SUCCESS]
Waiting: 100%|██████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [00:00<00:00, 396.06it/s, status=SUCCESS]
Waiting: 100%|███████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████████| 100/100 [00:05<00:00, 18.17it/s, status=SUCCESS]
Fetch the results in batches too:
[45]:
# get all of the results for the deletion region scan
# NOTE - empty string sequences are not scores by the PoET API
results_del_scan_dict = {}
for f in futures_score_del_span:
res = f.get()
for r in res:
results_del_scan_dict[r[1]] = r[2]
print('GET', len(results_del_scan_dict))
len(results_del_scan_dict)
GET 1900
GET 3758
GET 5092
[45]:
5092
[46]:
# match against the queries
scores_del_scan_ensemble = []
for q in queries:
s = results_del_scan_dict.get(q.decode(), np.array([np.nan]*10)) # fill in NaN for "" fragments
scores_del_scan_ensemble.append(s)
scores_del_scan_ensemble = np.stack(scores_del_scan_ensemble)
scores_del_scan_ensemble.shape
[46]:
(5417, 10)
[47]:
# visualize scores for the fragment deletions
max_length = 16
scores_mat = np.zeros((len(wt), max_length)) + np.nan
for i in range(len(wt)):
for j in range(i, min(i+max_length, len(wt))):
q = wt[:i] + wt[j+1:]
s = results_del_scan_dict.get(q.decode(), [np.nan]*10) # fill in NaN for "" fragments
s = np.array(s)
scores_mat[i, j-i] = s.mean() - baseline.mean()
scores_mat.shape
[47]:
(346, 16)
[48]:
_, ax = plt.subplots(figsize=(20, 8))
im = ax.imshow(scores_mat.T, cmap='viridis')
ax.set_xlabel('Position')
ax.set_ylabel('Deletion Size')
ax.set_title('Deletion Size Tolerance');

[49]:
# sort out the most favorable deletion starting from each position
order = np.argsort(-scores_mat.max(axis=1).ravel())
print('Score ', 'Start,End', 'Length', 'Sequence', sep='\t')
for k in order[:10]:
i = k
j = np.argmax(scores_mat[i])
#i, j = np.unravel_index(k, scores_mat.shape)
span = (i+1, i+1+j)
deleted_fragment = wt[i:i+j+1].decode()
print(f'{scores_mat[i, j]:8.5f}', span, j+1, deleted_fragment, sep='\t')
Score Start,End Length Sequence
13.03051 (np.int64(330), np.int64(345)) 16 AQCPVGRLPYEGLEKE
13.03051 (np.int64(331), np.int64(346)) 16 QCPVGRLPYEGLEKEA
8.18189 (np.int64(329), np.int64(344)) 16 VAQCPVGRLPYEGLEK
5.88019 (np.int64(327), np.int64(342)) 16 TGVAQCPVGRLPYEGL
4.11371 (np.int64(328), np.int64(342)) 15 GVAQCPVGRLPYEGL
3.78090 (np.int64(325), np.int64(340)) 16 STTGVAQCPVGRLPYE
3.56178 (np.int64(326), np.int64(341)) 16 TTGVAQCPVGRLPYEG
1.33338 (np.int64(321), np.int64(336)) 16 RLTRSTTGVAQCPVGR
0.49578 (np.int64(323), np.int64(337)) 15 TRSTTGVAQCPVGRL
0.49578 (np.int64(322), np.int64(336)) 15 LTRSTTGVAQCPVGR
[50]:
# sort out the most favorable deletion starting from each position
order = np.argsort(-scores_mat[:320].max(axis=1).ravel())
print('Score ', 'Start,End', 'Length', 'Sequence', sep='\t')
for k in order[:10]:
i = k
j = np.argmax(scores_mat[i])
#i, j = np.unravel_index(k, scores_mat.shape)
span = (i+1, i+1+j)
deleted_fragment = wt[i:i+j+1].decode()
print(f'{scores_mat[i, j]:8.5f}', span, j+1, deleted_fragment, sep='\t')
Score Start,End Length Sequence
0.35025 (np.int64(77), np.int64(89)) 13 VAIPGEETEIFSR
0.05758 (np.int64(62), np.int64(77)) 16 LQGIMYDPAEMMETAV
-0.16627 (np.int64(76), np.int64(89)) 14 AVAIPGEETEIFSR
-0.69624 (np.int64(75), np.int64(89)) 15 TAVAIPGEETEIFSR
-1.03163 (np.int64(318), np.int64(331)) 14 NVERLTRSTTGVAQ
-1.12549 (np.int64(284), np.int64(296)) 13 YSGLQASGDGDRG
-1.12549 (np.int64(283), np.int64(295)) 13 GYSGLQASGDGDR
-1.12549 (np.int64(282), np.int64(294)) 13 RGYSGLQASGDGD
-1.27347 (np.int64(285), np.int64(297)) 13 SGLQASGDGDRGL
-1.33994 (np.int64(316), np.int64(331)) 16 RENVERLTRSTTGVAQ
Deletion analysis summary#
We can see that PoET identifies that C-terminal deletions are most favorable and are predicted to be highly tolerated. Examining the protein on PDB, 2UXY, we can see that several C-terminal residues are missing from the crystal structure and that the remaining observed C-terminal residues form a relatively unstructured loop away from the active site, validating that this deletion would be plausibly tolerated.
Note that PoET sometimes assigns higher likelihoods to protein fragments, so large deletion scans should be performed with some caution. This is because shorter sequences contain fewer amino acids that need to be explained by the model and sequence fragments often occur in natural protein databases leading to some PoET tending to prefer early stop tokens.
Summary#
We have here used a combination of PoET (an OpenProtein proprietary model) and ESM, PSSM models (open-source) to look at sequence properties of aliphatic amidase. We have used PoET to examine every possible single site mutation and its predicted effect on fitness. Such an approach can be used to design novel mutants or find positions of interest. The flexibility of PoET also allows us to score variable length deletions, which seem to explain structural features of aliphatic amidase, further mutational analysis could verify these results.
Lastly, we have demonstrated that wetlab DMS data correlates well with our PoET scores (spearman r> 0.6 across 3 distinct properties), and that the DMS data is better explained by PoET (ranged of Spearman r = 0.63-0.67) than either PSSM (r = 0.49-0.5) and ESM1b (r= 0.44-0.59).
Overall, PoET is a powerful and flexible protein model to understand and exploit sequence constraints.